Abstract
Introduction: Shared decision-making is the heart of patient and family-centered care. The motivating drive behind all health care decisions should be the individual health care needs and desired health outcomes of the patient. Older patients with acute myeloid leukemia (AML) are faced with a life-threatening cancer and limited time to make complex decisions regarding treatment. A key component of the shared decision-making process is the patient's goals with treatment: survival (even if quality of life (QOL) is compromised), or improved QOL (with potential for shortened survival). The objective of this analysis is to provide demographic characteristics and goals of treatment, along with alignment between goals of treatment and actual treatment received, from the first 135 patients in the development of a patient decision-making model comparing intensive with non-intensive treatment.
Methods: Following institutional review board approval, this single center prospective observational study at Moffitt Cancer Center began recruitment of newly diagnosed AML patients 60+ years of age on July 8, 2020. Baseline demographics and treatment goals were obtained from each patient within seven days of beginning AML treatment. Patients were asked to choose between improved survival or QOL as their primary treatment goal.
Results: Our study included 135 newly diagnosed AML patients 60+ years of age who were within seven days of starting treatment for AML. Treatment between intensive and non-intensive groups were 73 (54%) and 62 (45.9%), respectively. There were 87 men (64.4%) and 48 women (35.6%). White, non-Hispanics comprised 118 (87.4%) of participants. There were five (3.7%) White-Hispanics in the study, and six (4%) Black, non-Hispanic. Additional participants were three Ashkenazi Jews (2.2%) of European descent, and one (.7%) Chinese participant.
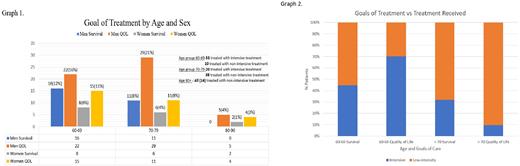
The age range of patients was 60-86 years. Treatment intensity varied between the 10-year age groups. Most patients, 53(84%), in the 60-69 age group, were treated with intensive treatment. The 70-79 age group included 20 patients treated with intensive treatment and 38 patients treated with non-intensive treatments. All subjects in the 80+ age group were treated with non-intensive treatment. The most frequent intensive treatment was CPX 351, and the most common non-intensive treatment was Azacytidine and Venetoclax.
Eighty-six patients (64%) chose QOL over survival. Five patients did not identify a goal of treatment. Treatment goals by decade and sex did not differ (Graph 1). Treatment intensity did not align with goals of treatment for both the younger and the older age groups with younger patients with survival goals receiving non-intensive therapy and older patients interested in survival receiving non-intensive treatment. (Graph 2). This shows the discordance between patient defined goals of treatment and treatment received.
Conclusions: Individual patient treatment goals must be identified to provide goal concordant care. Quality of life data are limited between treatment intensities. Current AML treatment intensity selection is primarily based on survival data, without formal consideration of impact of treatment on the quality of survival. Our data demonstrate the potential discordance between patient treatment goals, and treatment received. This underscores the need for a comprehensive clinical decision-making model which captures the goals of the patient, and provides detailed symptom and QOL data related to treatment options, intense or non-intense. Robust data is needed outlining individual disease characteristics and patient factors which predict optimal survival and QOL associated with treatment intensity. Patients identified that they value improvement in QOL over survival. Future development of an NIH funded QOL decision-making model for older patients with AML based on disease characteristics and patient factors will provide guidance to better align treatment with patient goals of treatment.
Disclosures
Tinsley-Vance:Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; CTi: Consultancy, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Chan:Syntrix Pharmaceuticals: Research Funding. Padron:Kura: Research Funding; Taiho: Honoraria; BMS: Research Funding; Blueprint: Honoraria; Stemline: Honoraria; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding. Sallman:Intellia: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Nemucore: Membership on an entity's Board of Directors or advisory committees; Lixte: Patents & Royalties: LB-100; Syntrix Pharmaceuticals: Research Funding; Syndax: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Takeda: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees. Sweet:berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Boxer Capital: Consultancy; Agios/Servio: Consultancy; Dava Oncology: Consultancy; Syntrix Pharmaceuticals: Research Funding; Jasper Therapeutics: Consultancy; Novartis: Consultancy; Dedham Group: Consultancy; Astellas: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Komrokji:Servier: Consultancy, Honoraria, Speakers Bureau; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Acceleron Pharma: Consultancy; Geron: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal